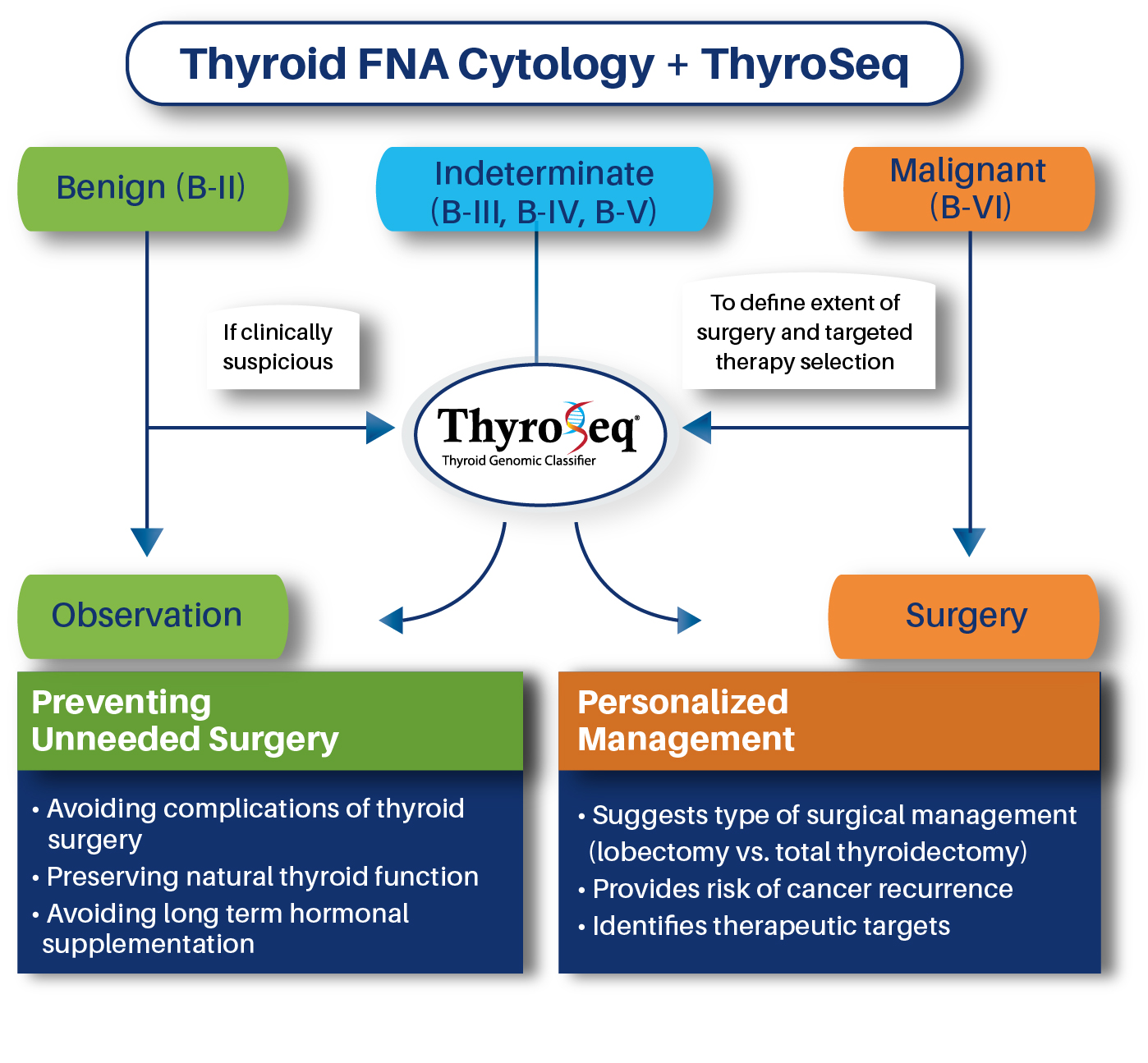
Diagnostic Utility
The primary application of ThyroSeq® is to provide accurate cancer diagnosis in thyroid nodules with indeterminate FNA cytologyUncertain risk of cancer in thyroid nodules with indeterminate FNA cytology (Bethesda categories III, IV, and V) hampers clinical management of these patients. ThyroSeq® stratifies these nodules into those that are likely benign and may be observed and those that are likely malignant and require surgical management. For the latter nodules, ThyroSeq may inform the extent of surgical management (lobectomy vs. total thyroidectomy)
In addition, ThyroSeq may help to clarify diagnosis in benign cytology nodules (Bethesda II) with clinical suspicion for malignancy
Superior Performance in Hürthle Cell Nodules
ThyroSeq® uniquely detects chromosomal copy number alterations, which are a hallmark of Hürthle cell cancer 1,2
Data from the multicenter clinical validation study and an independent real-world study show reliable stratification of Hürthle cell nodules with as high as 100% sensitivity, 67% specificity, 100% NPV and 64% PPV 3,4
The majority of patients with Hürthle cell nodules can safely avoid surgery with ThyroSeq® 4
Accurately Diagnose Medullary Thyroid Carcinoma and Parathyroid Nodules
Approximately 1% of medullary thyroid carcinoma (MTC) and parathyroid nodules are misdiagnosed on cytology as follicular thyroid cell lesions
In one test, ThyroSeq® provides highly sensitive detection of MTC3, 6-8 and parathyroid nodules5,6,9
References:
1.Ganly I, et al. Cancer Cell. 2018. 2.Gopal RK, et al. Cancer Cell. 2018. 3.Steward DL, et al. JAMA Oncol. 2018. 4.Schatz-Siemers N, et al. Diagn Cytopathol. 2019. 5.Nikiforova MN, et al. Cancer Cytopathol. 2020. 6.Nikiforova MN, et al. Cancer. 2018. 7.Carty SE, et al. Ann Surg. 2020. 8.Abdelhakam DA, et al. JASC. 2020. 9.Cho M, et al. Cancer Cytopathol. 2017.
