Cancer Prognostication and Therapy
Comprehensive molecular profiling by ThyroSeq® provides pre-operative prognostication of cancerous nodules, informing the extent of surgery and therapeutic options
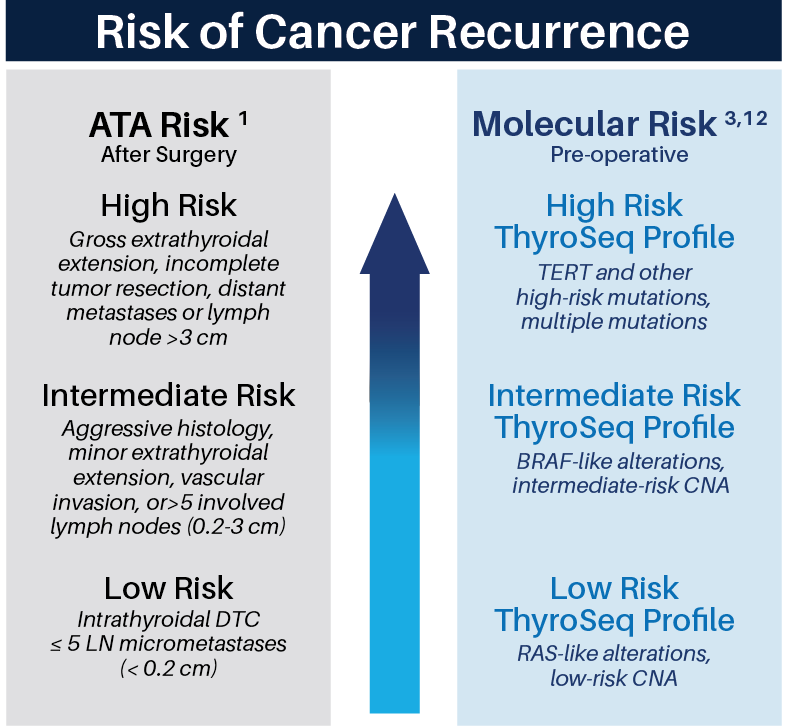
Based on the management guidelines from the American Thyroid Association (ATA), thyroid cancer risk stratification is important for selecting the appropriate extent of surgery (lobectomy vs total thyroidectomy), radioactive iodine (RAI) treatment, and intensity of follow-up. Most thyroid cancers are indolent and these patients are at low risk for disease recurrence after cancer removal. These patients can be treated by lobectomy and are unlikely to benefit from RAI ablation and TSH suppression. On the other hand, patients with high-risk cancers would benefit from up-front total thyroidectomy, which facilitates post-operative RAI administration and disease monitoring. ThyroSeq® provides most complete pre-operative assessment of risk of cancer recurrence in patients with thyroid nodules.
ThyroSeq® Cancer Risk Classifier (CRC)
ThyroSeq v3 Cancer Risk Classifier is developed to be used in FNAs from cytologically malignant (Bethesda VI) nodules and in resected thyroid cancers (formalin fixed paraffin-embedded (FFPE)) to assist in cancer risk stratification and patient management. Similar to ThyroSeq v3 Genomic Classifier which is used for nodules with indeterminate cytology (Bethesda III-V), it utilizes a next-generation sequencing of DNA and RNA from 112 genes. However, instead of prediction of probability of cancer in tested nodule, the main focus of the test is to predict risk of cancer recurrence required for selecting the extent of surgical management and further therapeutic options for patients with thyroid cancer. The findings are reported as Low, Intermediate or High risk of Cancer Recurrence.
ThyroSeq® provides assessment of the Risk of Cancer Recurrence (CRC) based on:
- Detection of all known molecular alterations associated with thyroid cancer aggressiveness (TERT, TP53, AKT1, PIK3CA and others) 2
- Case-control study of 287 patients either with distant metastasis or without distant metastasis on ≥5 years of follow-up 12
- Prognostic information contained in the proprietary database of >3,000 thyroid nodules with known surgical outcome 9
- Assessment of cancer risk and evaluation of aggressive thyroid cancers in studies that utilized ThyroSeq 3,10-12
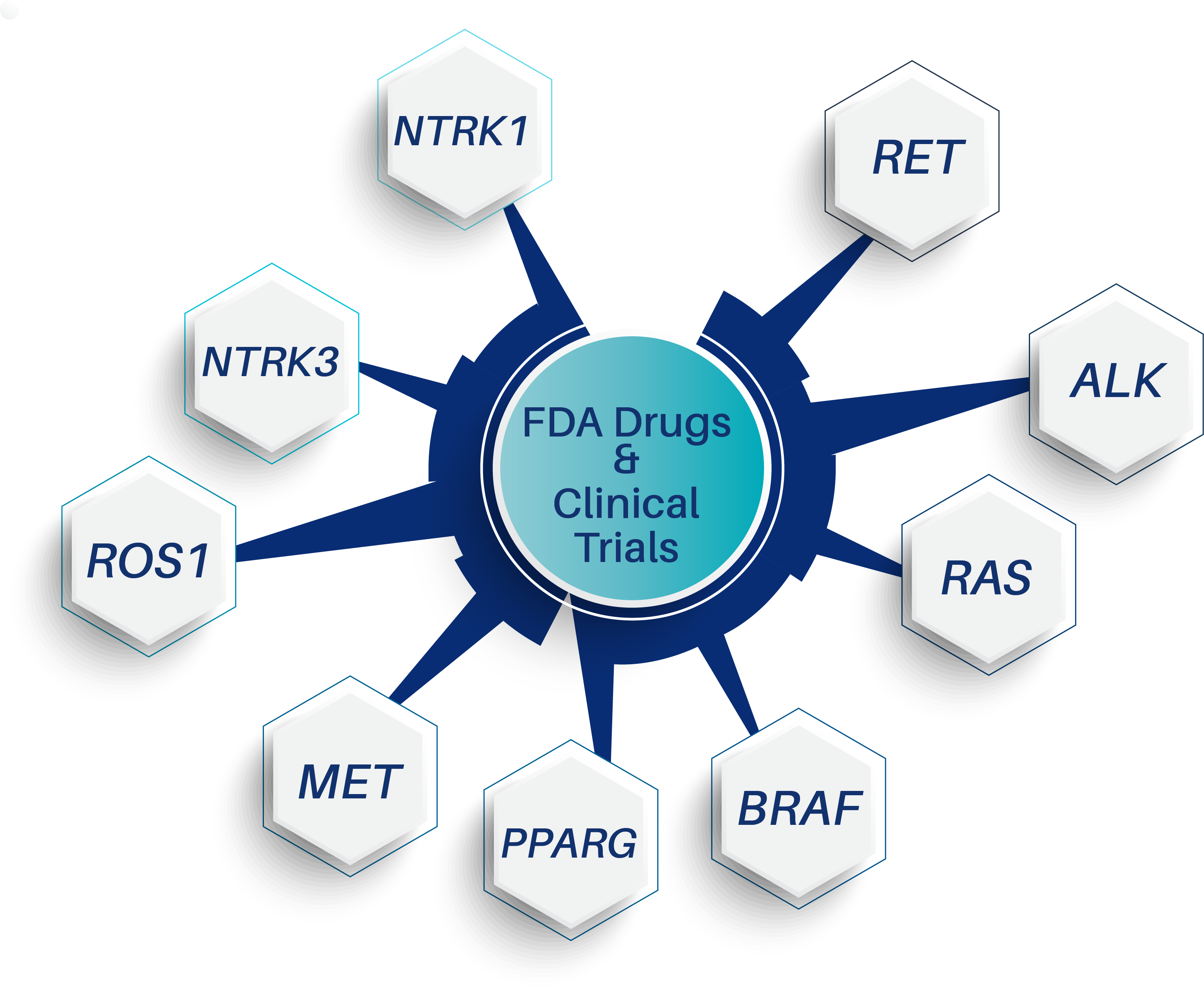
Therapeutic gene targets (variants and fusions) detectable by ThyroSeq
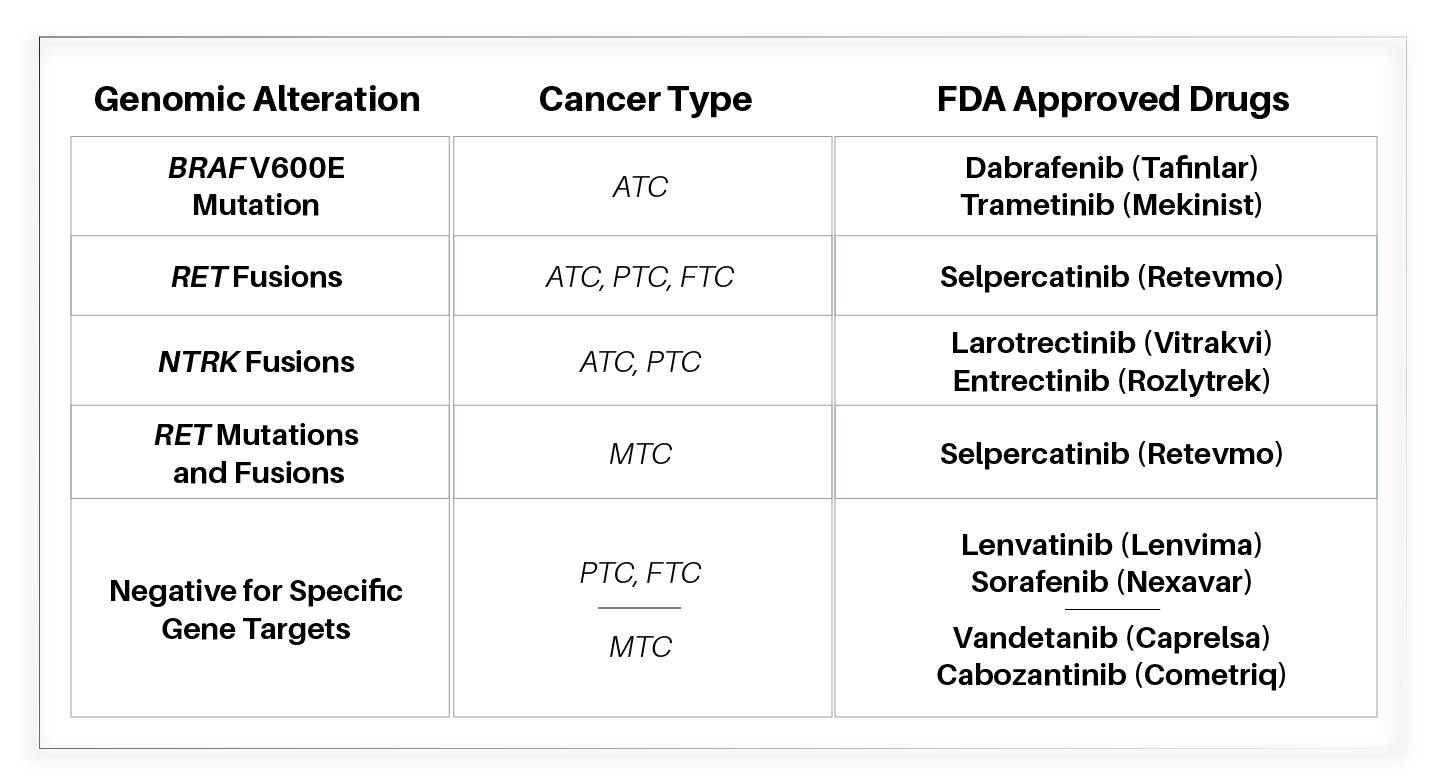
ThyroSeq® Detects Clinically Actionable Therapeutic Targets
- In advanced thyroid cancer, ThyroSeq offers the detection of therapeutic targets for FDA approved drugs or enrollment into clinical trials that actively recruit patients 2, 13-16
- Every ThyroSeq report contains information matching the detected gene target with corresponding treatment options
- For anaplastic thyroid carcinoma, ThyroSeq provides rapid detection of the BRAF V600E mutation critical for effective treatment
FDA Approved Targeted Therapies for Advanced Thyroid Cancer*
Abbreviations: ATC, anaplastic thyroid carcinoma; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; MTC, medullary thyroid carcinoma
*Learn more about FDA approved targeted therapy drugs for advanced thyroid cancer at American Cancer Society website
Thyroid Cancer Clinical Trials
Learn more about thyroid cancer clinical trials:
|
International Thyroid Oncology Group (ITOG), a multidisciplinary team of leading physicians, scientists, and advocates to design, coordinate, and prioritize state-of-the-art clinical trials and correlative science. |
|
ClinicalTrials.gov is a web-based resource that provides patients, their family members, health care professionals, researchers, and the public with easy access to information on publicly and privately supported clinical studies |
Supporting articles:
3. Prognostic of recurrence and survival in poorly differentiated thyroid cancer - PubMed (nih.gov)
References:
1. Haugen BR, et al. Thyroid. 2016. 2. Nikiforova MN, et al. Cancer. 2018. 3. Chin PD, et al. Endocrin Pathol. 2020. 4. Xing M, et al. J Clin Oncol. 2015. 5. Song YS, et al. Cancer. 2016. 6. Ganly I, et al. Cancer Cell. 2018. 7. Gopal RK, et al. Cancer Cell. 2018. 8. Nikiforov YE. Endocr Pract. 2017. 9. UPMC, data on file. 10. Yip L, et al. Ann Surg. 2015. 11. Chernock RD, et al. Mod Pathol. 2020. 12. Yip et al Cancer (2021) 127(11):1779-1787]. 13. Panebianco F, et al. Endocr Relat Cancer. 2019. 14. Seethala RR, et al. Am J Surg Pathol. 2017. 15. Kelly LM, et al. Proc Natl Acad Sci U S A. 2014. 16. Nikiforov YE. Endocr Pathol. 2002.