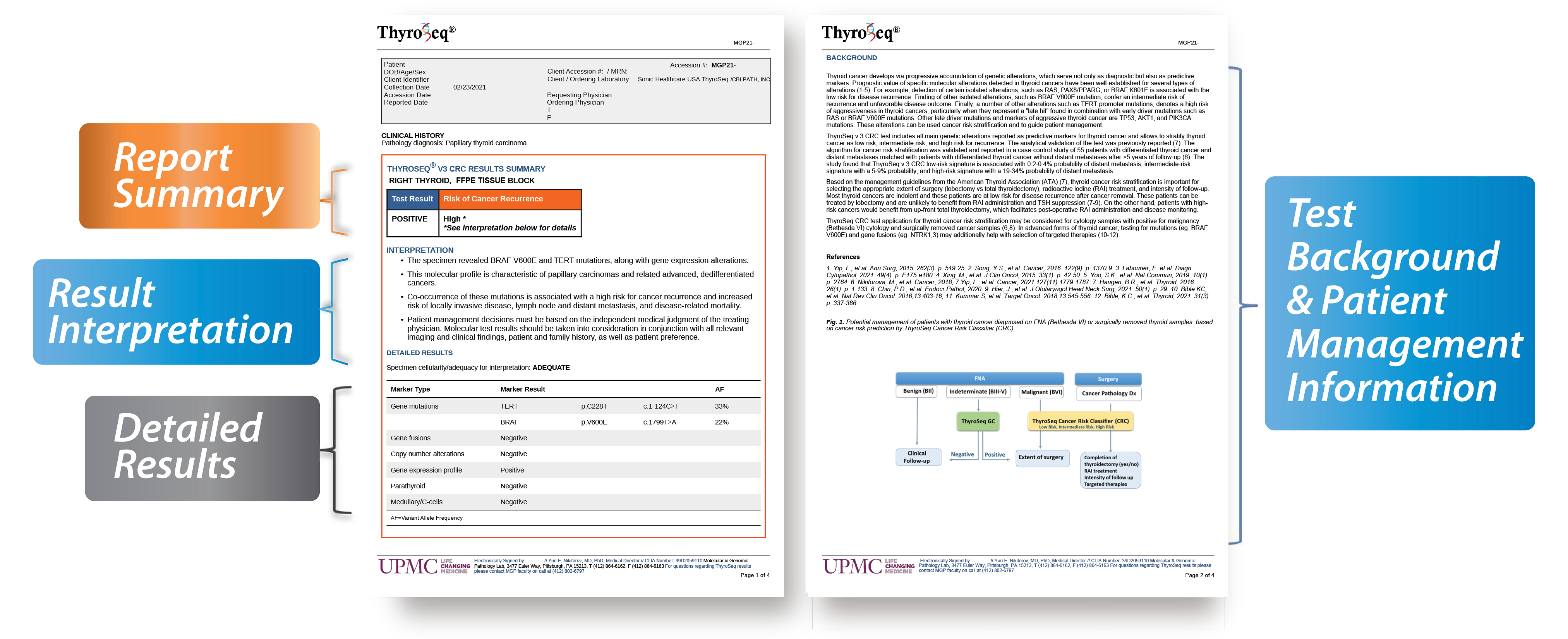
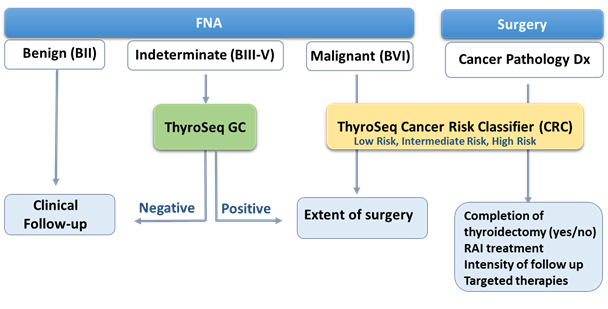
ThyroSeq® Cancer Risk Classifier (CRC) Sample Report
The results of the ThyroSeq® v3 CRC test are reported in a user-friendly format that contains:
- Report Summary with an assessed risk of cancer recurrence based on detected molecular alterations in the patient's FNA sample from cytologically malignant (Bethesda VI) nodule or resected thyroid cancer
- Result Interpretation section providing more information on types of genetic alterations detected, their association with a specific type of cancer and with the risk of cancer recurrence, suggested potential clinical management, including selection of targeted therapy
- Detailed Results section lists all alterations detected including mutation allele frequency and fusion gene partners